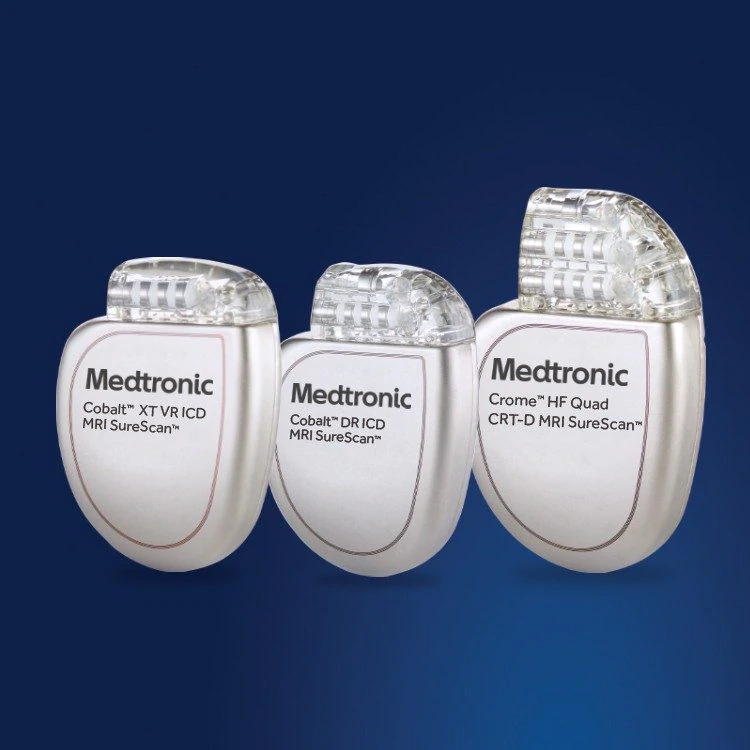
![Medtronic Blue Pacemaker [13] System. From left to right, the image... | Download Scientific Diagram Medtronic Blue Pacemaker [13] System. From left to right, the image... | Download Scientific Diagram](https://www.researchgate.net/publication/341907316/figure/fig1/AS:898684128604160@1591274401110/Medtronic-Blue-Pacemaker-13-System-From-left-to-right-the-image-shows-a-programmer-a.jpg)
Medtronic Blue Pacemaker [13] System. From left to right, the image... | Download Scientific Diagram

Medtronic snags another Class I recall, this time for its artery-clearing HawkOne system | Fierce Biotech

PDF) Design of the Pacemaker REmote Follow-up Evaluation and Review (PREFER) trial to assess the clinical value of the remote pacemaker interrogation in the management of pacemaker patients